Clinical Trials 101
Frequently Asked Questions
What are clinical studies?
Clinical studies are research studies in which real people participate as volunteers. Clinical research studies are a means of improving our understanding of disease, such as in observational studies, or developing new treatments and medications for diseases and conditions, such as clinical trials, which are evaluating the effects of a biomedical or behavioral intervention on health outcomes. There are strict rules for clinical trials, which are monitored by the National Institutes of Health for the trials it funds, and the U.S. Food and Drug Administration more broadly. Some of the research studies at the Clinical Center involve promising new treatments that may directly benefit patients.
Why should I participate?
The health of millions has been improved because of advances in science and technology, and the willingness of thousands of individuals like you to take part in clinical research. The role of volunteer subjects as partners in clinical research is crucial in the quest for knowledge that will improve the health of future generations.
Source: MS Research Australia
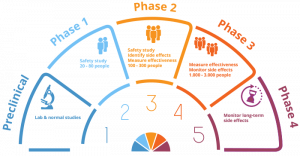
What are Phase I, Phase II, Phase III, and Phase IV studies?
Phase 1 studies are used to learn the "maximum tolerated dose" of a drug that does not produce unacceptable side effects. Patient volunteers are followed primarily for side effects, and not for how the drug affects their disease. The first few volunteer subjects receive low doses of the trial drug to see how the drug is tolerated and to learn how it acts in the body. The next group of volunteer subjects receives larger amounts. Phase 1 studies typically offer little or no benefit to the volunteer subjects.
Phase 2 studies involve a drug whose dose and side effects are well known. Many more volunteer subjects are tested, to define side effects, learn how it is used in the body, and learn how it helps the condition under study. Some volunteer subjects may benefit from a phase 2 study.
Phase 3 and 4 studies compare the new drug against a commonly used drug. Some volunteer subjects will be given the new drug and some the commonly used drug. The trial is designed to find where the new drug fits in managing a particular condition. Determining the true benefit of a drug in a clinical trial is difficult.
Placebos are inactive substances made to look like the real medicine used in the clinical trial. Placebos allow the investigators to learn whether the medicine being given works better or no better than ordinary treatment. In many studies, there are successive time periods, with either the placebo or the real medicine. In order not to introduce bias, the patient, and sometimes the staff, are not told when or what the changes are. If a placebo is part of a study, you will always be informed in the consent form given to you before you agree to take part in the study. When you read the consent form, be sure that you understand what research approach is being used in the study you are entering.
What is the placebo effect?
Medical research is dogged by the placebo effect, the real or apparent improvement in a patient's condition due to wishful thinking by the investigator or the patient. Medical techniques use three ways to rid clinical trials of this problem. These methods have helped discredit some previously accepted treatments and validate new ones. Methods used are the following: randomization, single-blind or double-blind studies, and the use of a placebo.
What is randomization?
Randomization is when two or more alternative treatments are selected by chance, not by choice. The treatment chosen is given with the highest level of professional care and expertise, and the results of each treatment are compared. Analyses are done at intervals during a trial, which may last years. As soon as one treatment is found to be definitely superior, the trial is stopped. In this way, the fewest number of patients receive the less beneficial treatment.
What are single-blind and double-blind studies?
In single- or double-blind studies, the participants don't know which medicine is being used, and they can describe what happens without bias. Blind studies are designed to prevent anyone (doctors, nurses, or patients) from influencing the results. This allows scientifically accurate conclusions. In single-blind ("single-masked") studies, only the patient is not told what is being given. In a double-blind study, only the pharmacist knows; the doctors, nurses, patients, and other health care staff are not informed. If medically necessary, however, it is always possible to find out what the patient is taking.
Are there risks involved in participating in clinical research?
Risks are involved in clinical research, as in routine medical care and activities of daily living. In thinking about the risks of research, it is helpful to focus on two things: the degree of harm that could result from taking part in the study, and the chance of any harm occurring. Most clinical studies pose risks of minor discomfort, lasting only a short time. Some volunteer subjects, however, experience complications that require medical attention. The specific risks associated with any research protocol are described in detail in the consent document, which you are asked to sign before taking part in research. In addition, the major risks of participating in a study will be explained to you by a member of the research team, who will answer your questions about the study. Before deciding to participate, you should carefully weigh these risks. Although you may not receive any direct benefit as a result of participating in research, the knowledge developed may help others.